We have been at the forefront of developing and delivering clinical trial and commercial GMP cell-based products since 2003. We have experience and expertise across a wide range of cell types, from mesenchymal stromal cells to chimeric antigen receptor (CAR) T-cells and offer full “needle-to-needle” process control – from patient cell collection back to reinfusion to the same patient.
Contract Manufacturing
Your partner for delivering cell and gene therapy products for global programs.

Contract Manufacturing expertise from over 20 years in the sector
Needle-to-Needle Control
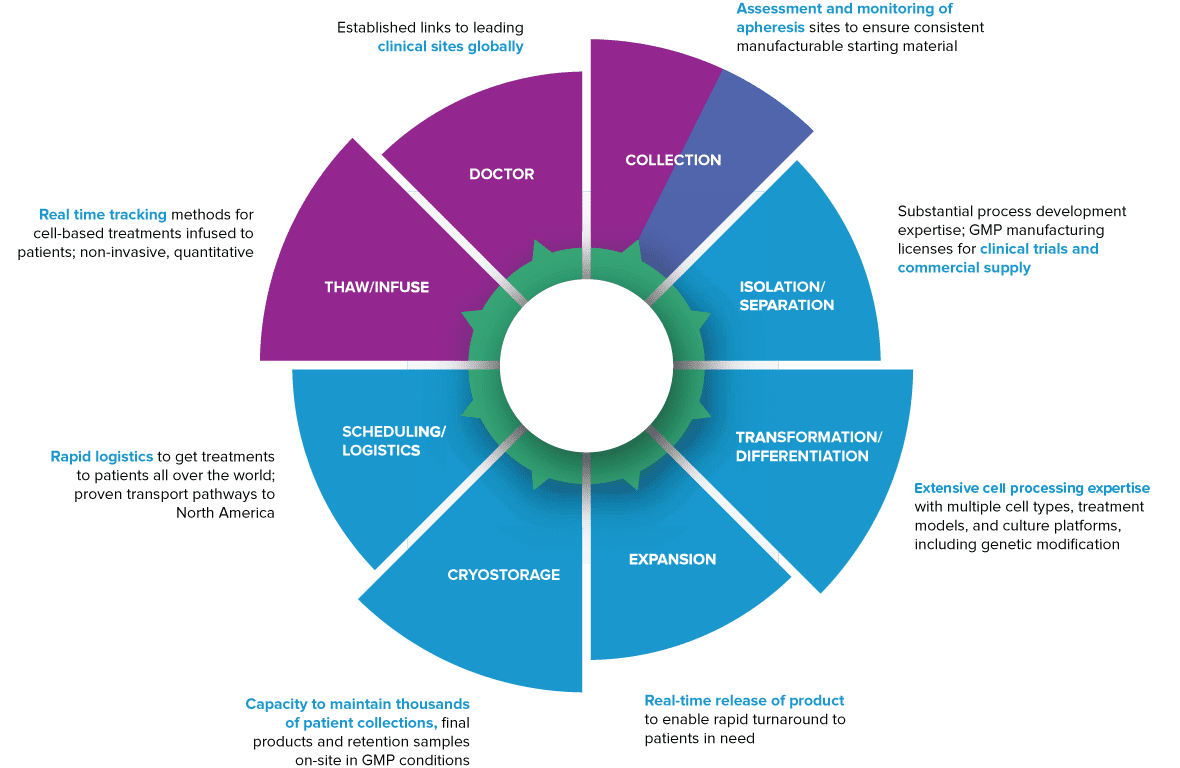
Explore our manufacturing capabilities
Our experience in GMP manufacturing of complex and sophisticated cell-based therapies can support your global program.
Process development and optimization
Ranging from process flexibility and rapid process scale-up for early-stage clinical products, to cost reduction and standardization for late-stage and commercial products
Technology Transfer
Efficient translation of client requirements into and out of our manufacturing facility, including testing, documentation, and process flows to ensure successful translation between sites
Storage and Distribution Logistics
Validated liquid nitrogen storage of cell therapy products to meet regulatory requirements, and distribution partnerships to ensure global critical-product delivery
Explore our services
Cell Therapies has extensive experience in the development and commercialization of cell and gene therapies for the Australian and global markets.
Apheresis Management
We can help you with apheresis collection protocol development, site selection and assessment, network management, cryostorage, and distribution
Clinical Trial Support
We manufacture products for clinical trial, provide process development for programs, manage product storage, and supply logistics and distribution
Consulting and Advisory
Our experts advise on Chemistry, Manufacturing, and Control (CMC), Australian and international regulatory standards and requirements, and clinical program development

